Essential oils are highly concentrated, hydrophobic liquids that contain volatile compounds naturally found in plants. These oils, often referred to as volatile oils, ethereal oils, or simply plant oils, capture the unique aroma and properties of the source plant.
The most common method for extracting these oils is distillation, with Steam Distillation being widely used for its efficiency and preservation of delicate plant compounds. Our offerings include two primary methods for producing essential oils: the Steam Distillation Unit and the Vacuum Distillation Unit.
These distillation units come in a variety of vessel sizes, including 10, 20, 50, 100, and 200 liters, making them suitable for both atmospheric and full vacuum operations. Each unit is designed to ensure optimal extraction efficiency, enabling the production of high-quality essential oils consistently.
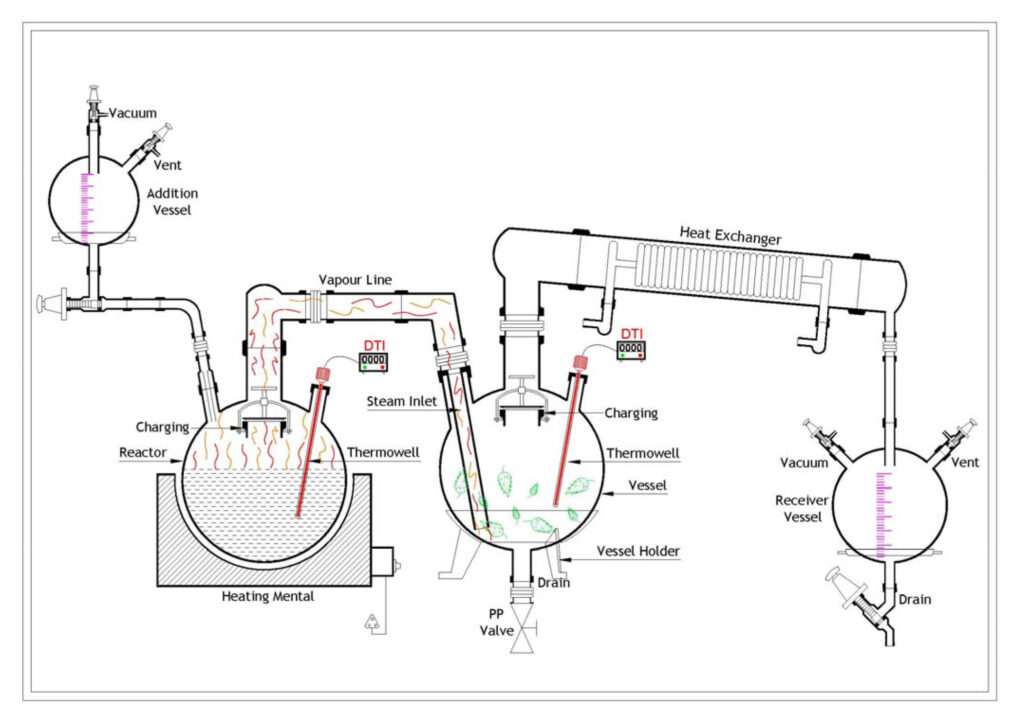
Steam distillation as a whole is a separation process which consists of distilling water together with other volatile and non‐volatile components. The water is heated up in the spherical vessel separately and the steam from the boiling water goes into the reactor where it reacts with the masses of trees and plants and further carries the vapor of the volatiles to a condenser, where both are cooled and return to the liquid or solid state; while the non‐volatile residues remain behind in the reactor.
|
Unit Cat. Ref. |
Reactor Capacity |
Mantle KW |
Addition Vessel |
Condenser HTA (M2) |
Receiver Vessel |
|
EOD 10 |
10 L |
1 KW |
5 L |
0.35 m2 |
5 L |
|
EOD 20 |
20 L |
1.8 KW |
5 L |
0.50 m2 |
5 L |
|
EOD 50 |
50 L |
3.6 KW |
20 L |
1.50 m2 |
20 L |
|
EOD 100 |
100 L |
5.4 KW |
20 L |
1.50 m2 |
20 L |
|
EOD 200 |
200 L |
8.1 KW |
50 L |
2.25 m2 |
50 L |

Vacuum distillation is a distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. A reduced pressure decreases the boiling point of compounds. The steam from the boiling water carries the vapor of the volatiles to a condenser, where both are cooled and return to the liquid or solid state; while the non‐volatile residues remain behind in the boiling container.
